
George Gassner
Styrene: Integral to the Global Economy but a Problem for Human Health
Styrenes plays a leading role in the global economy of the chemical industry where they serves as versatile building blocks in the synthesis of plastic, rubber, materials, and serve as integral component of the hydraulic fracturing fluids used in the mining of petroleum resources. Large-scale annual production and distribution of styrene results in widespread exposure and pollution that represents a significant human and environmental health concern. The toxicology of styrene manifests immediately as a biological membrane disruptor and neurotoxin. Beyond this immediate toxicity, intracellular epoxidation and hydroxylation styrenes by isoforms of cytochrome P450 results the production of vinyl phenols and styrene oxides correlated with oxidative stress and DNA-damage. The transformation of styrenes by intracellular metabolism to compounds that present a greater biologic threat to the cell than styrene itself leads styrene to be viewed as a Trojan Horse of hydrocarbon metabolism in human cells.

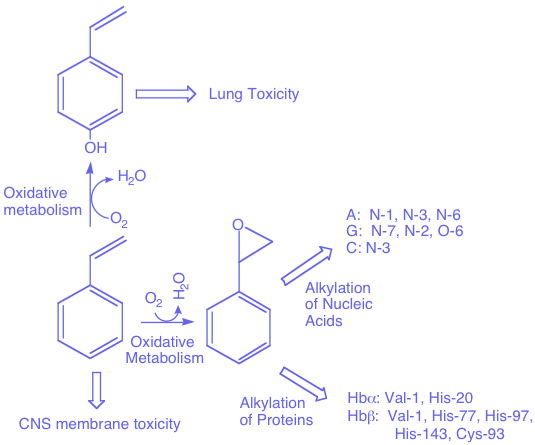
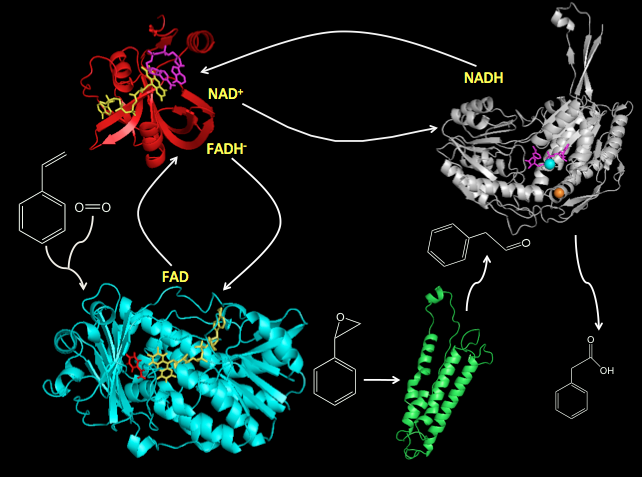
Mechanism Structure and Engineering of Styrene Catabolic Enzymes
Our studies engage SF State Chemistry and Biochemistry students in basic research studies of the structure and mechanism styrene catabolic and detoxification pathways of bacteria that have the unique ability to use styrene as a sole source of carbon and energy. The bacterial transform styrene in solvent-contaminated aqueous and terrestrial environments into cellular biomass represents an effective means of eliminating environmental pollution. Our basic research studies help to elucidate enzyme mechanisms that represent the molecular basis of oxidative styrene metabolism. The findings of this work contribute to immediate objectives of understanding the mechanisms involved in the cellular biochemistry of styrene metabolism and provide foundations for the development of enzyme-based strategies for environmental remediation and the development and engineering of enzymes as biocatalysts for the synthesis of products of biotechnological value.
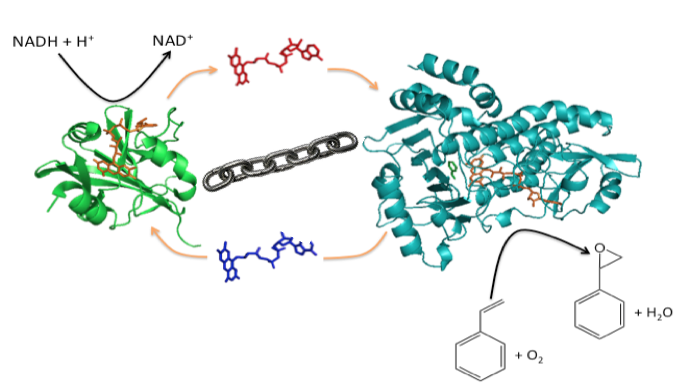
Styrene Monooxygenases (SMO) Enantioselective Synthesis of Styrene Oxides
The two-component Flavoprotein SMO occurs the entry point of the styrene catabolic and detoxification pathway. The structural and mechanistic basis of the enantioselective epoxidation of styrene to (S)-styrene oxide catalyzed by this two-component flavoprotein represents a central research focus of our laboratory. We are also working to elucidate regulatory mechanisms involved in the reduction and oxygenation reactions of styrene monooxygenases, and to engineer the active sites of these enzymes to better accommodate styrene analogs that vary in steric bulk and in the development of fusion proteins that join the reductase and monooxygenase domains in single polypeptide. Additional investigations focus on mechanisms of substrate channeling, protein-protein, protein-subunit interactions, and coenzyme recycling mechanisms involved in the mechanism of SMO.
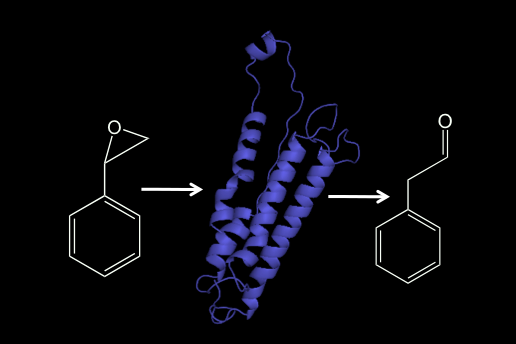
Styrene Oxide Isomerase (SOI)
SOI catalyzes the isomerization of styrene oxide to phenylacetaldehyde in the second step of the styrene catabolic pathway. We have expressed and studied the catalytic mechanism of SOI, which most likely occurs as a Type-1 integral membrane protein. We are interested in gaining a better understanding of the structure, active site features of SOI and in establishing how it interacts with the other components of the styrene pathway, which occur as soluble, cytosolic proteins.
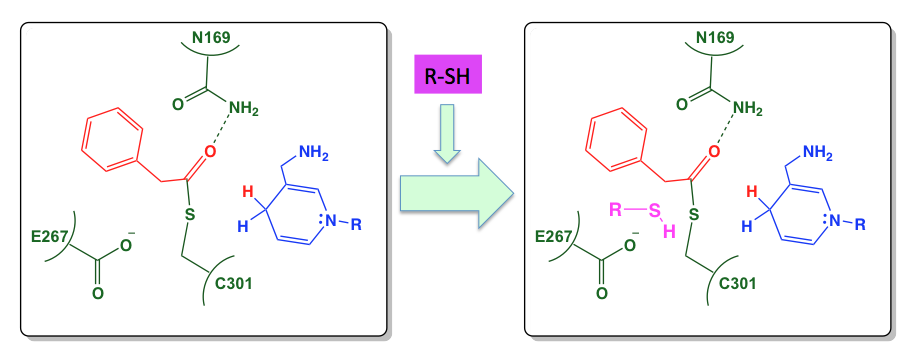
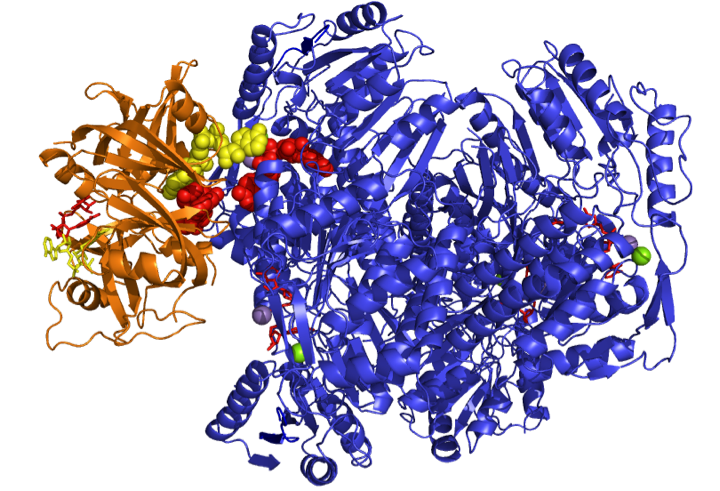
Phenylacetaldehyde Dehydrogenase (PADH)
PADH occurs at the interface of bacterial styrene and phenylacetic acid metabolism where it catalyzes the oxidation of phenylacetaldehyde to phenylacetate. We are currently investigating the structural and mechanistic basis of the aldehyde, oxoester, and thioester specificities PADH and how structural and mechanistic features of this enzyme alternately direct it toward the hydrolysis or synthesis of thioesters.
Approach to Experimental Biochemistry in the Gassner Lab
Studies completed in this lab are based on the enzymes that we recover by using protein purification techniques after overexpression from recombinant vectors. Experimental studies of purified proteins involve recording and analyzing model-based equilibrium, steady-state, and pre-steady state stopped-flow absorbance and fluorescence spectroscopic data. Students working in this lab are directly engaged in mentored training in all aspects of the work and develop expertise that allows them to actively engage in projects and actively experience the transition from the process of research to reward in new discovery. Undergraduate and MS students completing research in this lab typically go on to work productively in Bay Area Biotechnology or continue their education through MS, PhD, or professional school programs.
References
- Uchechi E. Ukaegbu, Auric Kantz*, Michelle Beaton*, George T. Gassner, and Amy C. Rosenzweig Structure and Ligand Binding Properties of the Epoxidase Component of Styrene Monooxygenase (2010) Biochemistry 49 1678-88.
- Auric Kantz* and George T. Gassner Nature of the Reaction Intermediates in the Flavin Adenine Dinucleotide-Dependent Epoxidation Mechanism of Styrene Monooxygenase (2011) Biochemistry, 50 523–532.
- Stephania Montersino, Dirk Tischler, George T. Gassner, and Willem J. H. van Berkel Catalytic and Structural Features of Flavoprotein Hydroxylases and Epoxidases (2011) Adv. Synth. Catal. 353, 2301–2319.
- Dirk Tischler, Michael Schlömann, Willem J.H. van Berkel, and George T. Gassner FAD C(4a)-hydroxide stabilized in a naturally fused styrene monooxygenase (2013) FEBS Letters, 587, 3848-3852
- Eliot Morrison, Auric Kantz, George T. Gassner, and Matthew H. Sazinsky Structure and Mechanism of Styrene Monooxygenase Reductase: New Insight into the FAD-Transfer Reaction (2013) Biochemistry, 52, 6063-6075.
- George T. Gassner, Sophia Chu*, Lisa Cox*, Nonye Okonokwo*, Juan Pena*, Andrew Skinner*, Phu Truong*, Kristine Vilaneuava*, and Alessesadro Maggi*. Built-In Regulatory Mechanisms and Active Site Plasticity Open the Door for Engineering the Styrene Catabolic Pathway Biophysical Journal 106, no. 2 (2014): 596a.
- Nonye Okonokwo* and George T. Gassner Bioengineered Styrene Monooxygenase: Elucidating the Impact of the N-Terminus in the Efficiency and Regulation of the Flavin-Transfer Reaction. (2015): FASEB J. 29, no.1 S572.
- Gozde Ulas, T. Lemmin, Y. Wu, GT Gassner, and William F. DeGrado Designed Metalloprotein Stabilizes a Semiquinone Radical. (2016) Nat Chem. 4 354-359
- Anders G Crabo*, Baljit Singh*, Tim Nguyen*, Shahram Emami*, George T Gassner, Matthew H Sazinsky D. Structure and Biochemstry of Phenylacetaldehyde Dehydrogenase from Pseudomonas putida (S12). Archives of Biochemistry and Biophysics Archives of Biochemistry and Biophysics : ABB. , (2017), 616, p.47-58
- Thomas Heine, Kathryn Tucker, Nonye Okonkwo*, Berhanegebriel Asseffa*, Catleen Conrad, Anika Scholtissek, Michael Schlölman, George Gassner and Dirk Tischler Engineering Styrene Monooxygenase for Biocatalysis: Reductase-Epoxidase Fusion Proteins Applied Biochemistry and Biotechnology (2017), 181(4), p. 1590-1610
- Thomas Heine, Willem.J.H. van Berkel, George Gassner, Karl-Heinz van Pee, and Dirk Tischler, Two-Component FAD-Dependent Monooxygenases: Current Knowledge and Biotechnological Opportunities, (2018), Biology 7(3), p. 1-35.
- George Gassner Methods in Enzymology V.620 New Approaches for Flavin Catalysis (2019) (Ed. Bruce Palfey) Chapter 16, The Styrene Monooxygenase System p.424-452